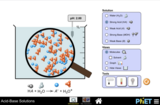
Learning Goals:Given acids or bases at the same concentration, demonstrate understanding of acid and base strength by: 1.Relating the strength of an acid or base to the extent to which it dissociates in water 2.Identifying all of the molecules and ions that are present in a given acid or base solution. 3.Comparing the relative concentrations of molecules and ions in weak versus strong acid (or base) solutions. 4.Describing the similarities and differences between strong acids and weak acids or strong bases and weak bases.Demonstrate understanding of solution concentration by: 1.Describing the similarities and differences between concentrated and dilute solutions. 2.Comparing the concentrations of all molecules and ions in concentrated versus dilute solutions of a particular acid or base.Use both the strength of the acid or base and the concentration of its solution in order to: 1.Describe in words and pictures (graphs or molecular drawings) what it means if you have a: Concentrated solution of a weak acid (or base) or Concentrated solution of a strong acid (or base) or other combinations. 2.Investigate different combinations of strength/concentrations that result in same pH values.Describe how common tools (pH meter, conductivity, pH paper) help identify whether a solution is an acid or base and strong or weak and concentrated or dilute.
- Subject:
- Chemistry
- Material Type:
- Activity/Lab
- Author:
- ERIN WOLFHOPE
- Date Added:
- 09/16/2021