This worksheet provides practice problems for computation of [H+] and [OH-].
- Subject:
- Science
- Material Type:
- Activity/Lab
- Provider:
- Chemistry 30
- Date Added:
- 10/11/2017

This worksheet provides practice problems for computation of [H+] and [OH-].

In this lab activity, students will determine the concentration of a solution of NaOH by titration with a standard solution of HCl. They will also determine the concentration of a sample of white vinegar by titration with a standard solution of NaOH.

In this lab activity, students will test the pH of a number of common substances.
This worksheet provides practice problems for computation of [H+] and [OH-].
This worksheet assesses students' understanding of reversible reactions, chemical equilibrium, and equilibrium constants.

This worksheet assesses students' understanding of shifts in equilibrium.
In this lab activity, students observe the effect of various stresses on equilibrium systems.
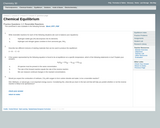
This worksheet assesses students' understanding of reversible reactions and chemical equilibirum.
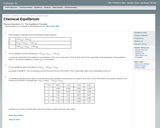
This worksheet assesses students' understanding of reversible reactions, chemical equilibirum, and equilibrium constants.
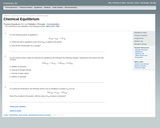
This worksheet assesses students' understanding of shifts in equilibrium.
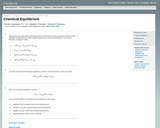
This worksheet assesses students' understanding of shifts in equilibrium.

In this lab activity, students will investigate some of the key factors that influence the rate of a reaction (particle size, temperature, concentration, catalysts).
In this lab activity, students will measure the temperature of a sample of a substance every 30 seconds as it undergoes changes of state. They will then graph the data for both the heating and cooling curve.
In this lab activity, students will use calorimetry to calculate the amount of heat absorbed or released during a simple reaction. Sample data is provided if students are not able to perform the actual experiment. A link to a virtual experiment is also provided.
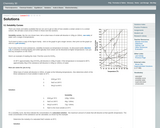
This resource explains to students how to analyze solubility graphs and provides practice exercises.
This worksheet provides practice problems related to solution concentrations.
In this lab activity, students will create a solubility curve for an ionic compound, potassium nitrate, using experimental data. Students will calculate the solubility of a substance under a variety of temperatures.
This worksheet assesses students' understanding of solution concentrations.
This worksheet assesses students' understanding of solution concentrations, especially as related to dilutions.

This worksheet assesses students' understanding of solutions. Students will need to use a solubility curve for common compounds to complete the worksheet.